AIDS is caused by human immunodeficiency viruses (HIV) that put immune cells— »T helper cells«—out of action. Instead of controlling other immune system cells in the defence against pathogens, infected T helper cells produce large quantities of new human immunodeficiency viruses. With the help of ultra-high-resolution imaging, an international research team including scientists from the University of Jena, has now managed to observe the spread of human immunodeficiency viruses amongst living T helper cells in real time.
The research team is using super-resolution STED fluorescence microscopy (see box on p. 55) to provide direct proof for the first time that the AIDS pathogen creates a certain lipid environment for replication. »This gives us a few starting points when it comes to investigating how to potentially prevent this reproduction,« says Prof. Dr Christian Eggeling, who conducts research and teaches at the Friedrich Schiller University Jena, the Leibniz Institute of Photonic Technology, and the University of Oxford.
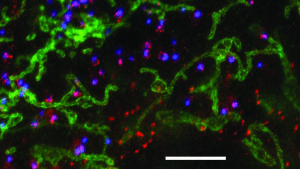
Together with his colleague Dr Jakub Chojnacki and a team of researchers directed by Prof. Dr Delphine Muriaux and Prof. Dr. Cyril Favard at the University of Montpellier Christian Eggeling hase been examining the plasma membrane of infected T helper cells. The researchers have been focusing on the »gate« through which the human immunodeficiency virus buds from the cell after multiplying inside. The »Gag« protein, which coordinates the processes involved in the assembly of newly produced virus particles, was used as a marker by tagging it with a green-fluorescent molecule. »Where this protein accumulates, the decisive processes take place that lead to the viruses releasing themselves and infecting other cells,« explains Christian Eggeling. In order to decifer these processes, the researchers examined the diffusion of the lipid molecules to and at the place where the »Gag« proteins gather, i.e. where the virus particle buds. During the budding process, the virus particles exit the cell through the plasma membrane and receive their lipid coating. By employing different types of red-fluorescent labeled lipids, Eggeling and his colleagues have now discovered that only certain lipids from the cell membrane interact with the human immunodeficiency virus. Although these lipids were already known, the research team has managed to directly observe this interaction in living cells for the first time.
STED microscopy enables super-resolution imaging. Here we can see the cellular components of fibroblast cells: mitochondria (green) and the peroxisomal protein PEX5 (red) on the surface of perixomes measuring approx. 200 nanometres (blue), which cannot be resolved with conventional confocal fluorescence microscopy. The white bar is five micrometres long (five thousandths of a millimetre). Adapted from »The Journal of Biological Chemistry« 291, 16948-16962, 2016.
Image: Christian Eggeling»This providesus with a potential target for antiviral drugs,« says Christian Eggeling. »Knowing which molecules the human immunodeficiency virus needs to exitthe cell and multiply is a crucial prerequisite for investigating how this can be prevented. Our technology enables us to follow the developments directly in real time«. Eggeling is now working with his team on the development of antibodies to attack precisely these molecules— and thus suppress the spread of the virus.
Tracking the movement of molecules in real time
»We not only want to examine these antibodies from a medical perspective, but also to find out how their biophysical interaction can be exploited to make them more effective,« states Eggeling. He is trying to understand how diseases develop on a molecular level by combining superresolution fluorescence microscopy with technology that tracks the movement of marked molecules in real time. This enables the spatial and temporal examination of individual molecules in living cells. »We can now reveal cellular mechanisms on a molecular level; these mechanisms were much too fast and occurred over far too small spatial scales for previous methods of investigation.«
By Lavinia Meier-Ewert
| STED stands for »stimulated emission depletion«. It is a method used in fluorescence microscopy that allows the optical resolution limit described by Ernst Abbe to be bypassed. Light is used to excite fluorescent dyes, which then spontaneously emit light. This spontaneous emission can be suppressed with the addition of high-intensity light at the wavelength range of the emission. This de-excitation light is placed in a ring around the focus of the sample to be examined, restricting the emission of fluorescent light to the centre of the sample. This optical trick makes the effective focal point significantly smaller, and its dimensions are below the Abbe diffraction limit. |
Original-Publikation: HIV-1 Gag specifically restricts PI(4,5)P2 and cholesterol mobility in living cells creating a nanodomain platform for virus assembly. Science Advances (2019), DOI: 10.1126/sciadv.aaw8651External link